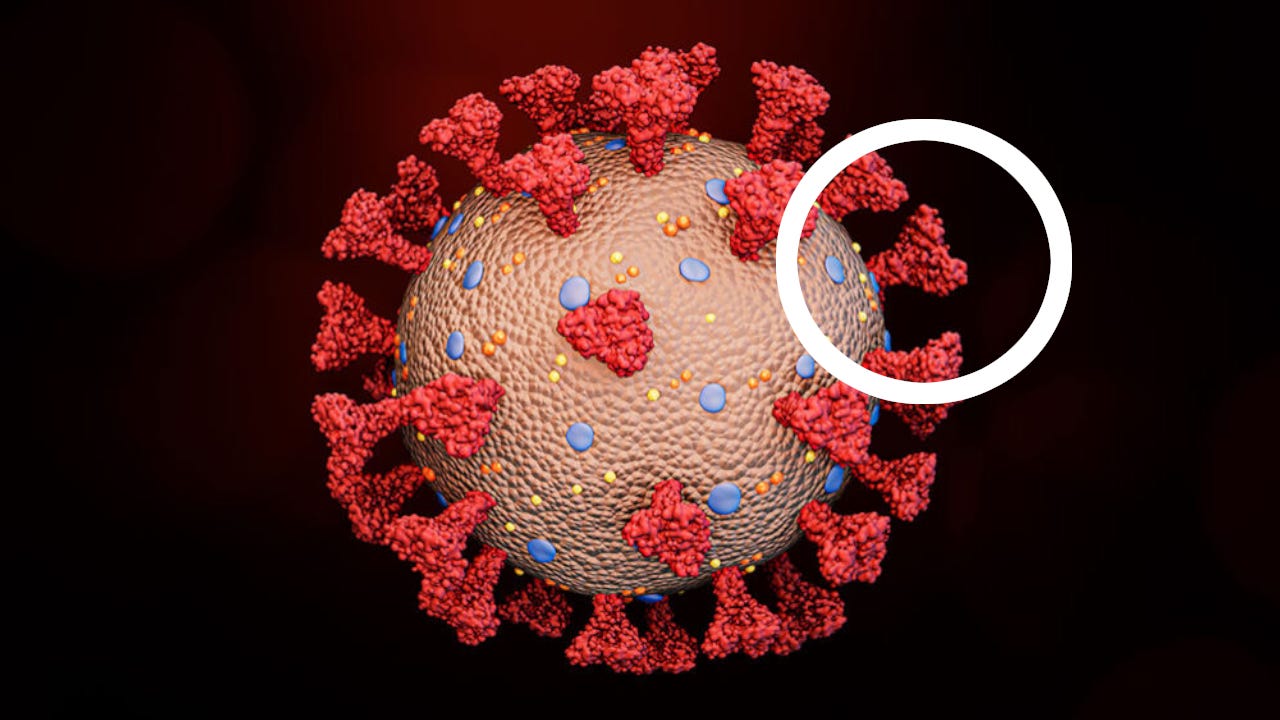
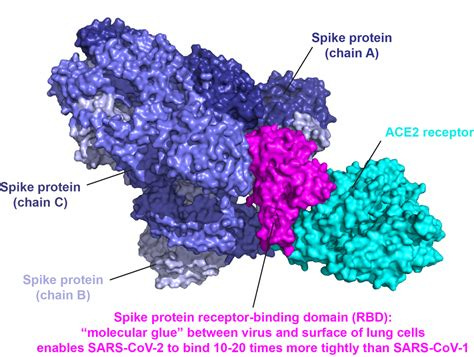
The spike protein is the portion of the coronavirus that attaches to ACE2 receptors found on cells throughout several major organ systems. Under normal conditions, ACE2 helps regulate blood pressure and fluid balance. During infection, however, the virus exploits this receptor as an entry point, binding tightly through the receptor-binding domain (RBD) of the spike protein to deliver its genetic material into the cell and trigger infection.
Since the rollout of the COVID-19 genetic injections, concerns about spike protein toxicity have become a subject of worldwide exploration. These injections deliver a payload of mRNA enclosed within lipid nanoparticles, which shield the genetic material from enzymatic degradation, and allow it to enter human cells via a Trojan horse effect. Once inside the cell, the nucleoside-modified mRNA engages the ribosomes, guiding them to synthesize the coronavirus spike protein. Under normal conditions, these ribosomes are responsible for producing the body’s own proteins, which are essential for healthy cellular function.
In the case of Pfizer injections, shots contain approximately 13 trillion mRNA molecules, while Moderna contains approximately 40 trillion. The quantity of total spike protein produced by the body in response to each shot has been immeasurable to date, and the duration of production also remains uncertain. Genomic integration remains an unsettling possibility, and recent findings from independent scientists have added weight to this growing concern.
When cells produce or become coated with these foreign proteins, the immune system can mistake healthy cells for invaders, triggering quasi-autoimmune reactions similar to organ rejection after a transplant. While this does not explain all of the mechanisms of disease linked to the COVID injections, this portion of the autoimmune disorders triggered does represent a large portion of the mechanisms. For more information on this matter, I strongly recommend this publication as a prerequisite:
Blocking spike proteins from binding to ACE2 receptors is therefore essential for recovery, prevention, and the preservation of long-term health. Let’s examine two safe and accessible molecules, which have demonstrated remarkable promise in blocking the spike protein from binding to ACE2 receptors through both in-silico studies and strong anecdotal evidence in successful clinical treatment.
Certain molecules can bind to the spike protein’s receptor-binding domain (RBD), preventing it from attaching to human cells. By blocking this interaction, the harmful effects associated downstream of RBD activity may be reduced. In vitro and computational studies also suggests that such compounds can potentially bind directly to the ACE2 receptor itself, occupying the site where the spike protein would normally latch on, thereby helping to protect cells from spike protein attachment.
In silico studies have shown that both ivermectin and quercetin can fit well into the spike protein’s RBD to ACE2 interface, effectively blocking its attachment to ACE2 receptors. This helps explain their reported benefits in preventing and treating COVID-19, as well as their potential to counter spike protein-related toxicity in individuals affected by the injections or by spike protein shedding.
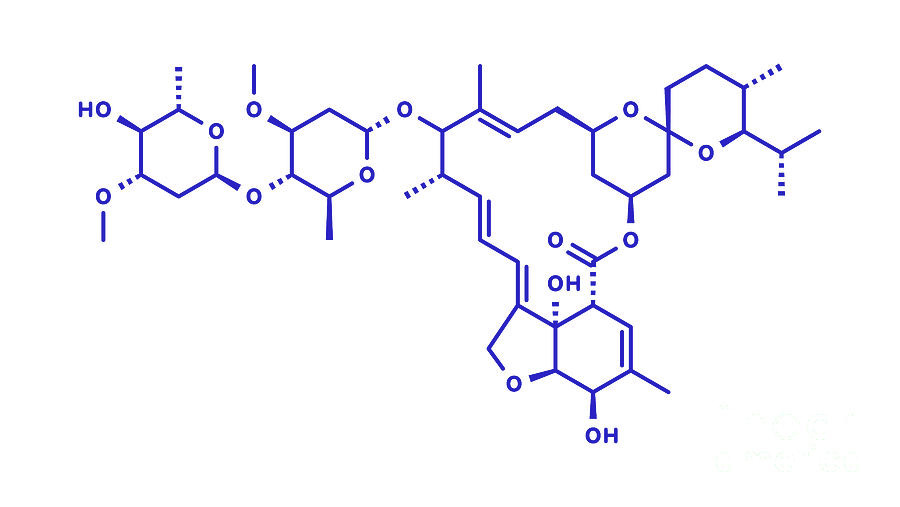
The Avermectin derivative Ivermectin compound has a long and respected history as an antiparasitic medicine used to treat infections caused by worms and other parasites. Ivermectin is linked to the portion of the 2015 Nobel Prize in Physiology or Medicine attributed to William C. Campbell and Satoshi Ōmura. It is routinely administered to animals and humans and has an excellent safety record.
Recent in vitro studies indicate that ivermectin exhibits antiviral activity against several viruses, including HIV 1, dengue, and SARS-CoV-2. Its antiviral mechanism involves interference with a cellular transport system known as the importin (IMP) complex, composed of the proteins importin-α and importin-β, which normally carry molecules into the cell nucleus. Many viruses exploit this pathway to move their proteins into the nucleus and suppress immune defenses. Ivermectin binds to importin-α, preventing it from pairing with importin-β, thereby blocking nuclear entry of viral proteins and inhibiting replication.
With its broad antiviral potential, affordability, and well-established safety record, ivermectin emerged as a practical alternative to the experimental and costly COVID-19 “vaccines” which actually had negative efficacy. Early in the pandemic, several regions in countries such as Utar Pradesh in India reported notable success using ivermectin for both prevention and treatment of COVID-19. However, widespread adoption of this inexpensive, off-patent medicine faced strong resistance from the pharmaceutical industry and its allies, as its use posed a direct challenge to the profitability of mRNA-based products.
In silico studies have shown that ivermectin binds strongly to the spike protein’s receptor-binding domain (RBD). Because of its relatively large molecular structure, ivermectin can potentially span multiple contact points on the RBD and may even interact with two RBDs simultaneously, making it a potent molecule for disabling the RBD-ACE2 interface of the spike protein. By occupying the site where the spike protein would normally bind, ivermectin blocks this interaction and helps to reduce spike-related cellular stress or immune reactions. While this represents one proposed mechanism by which ivermectin could interfere with spike protein activity, much remains to be explored about this remarkable compound as ongoing clinical observations and anecdotal reports continue to accumulate in exploration of its utility.
Quercetin is a natural plant flavonol found in fruits, vegetables, and herbs such as raw capers, red and yellow onions, kale, apples, fresh dill, dried chili pepper, and cilantro. In silico studies have shown that this compound can bind to the spike protein’s RBD–ACE2 interface, potentially blocking its ability to attach to the ACE2 receptor in a manner which is mechanistically similar to ivermectin.
Although quercetin operates through a mechanism similar to ivermectin, it is a smaller molecule—roughly one-third the size. This structural difference presents certain trade-offs. While quercetin appears less potent overall, computational studies suggest it binds to specific regions of the spike protein’s RBD that are relatively conserved across variants, which could make its effects less vulnerable to mutations of the spike protein. Additional advantages include its over-the-counter availability, excellent safety profile, and multifaceted benefits. Beyond its potential spike-blocking action, quercetin also exhibits strong antioxidant and anti-inflammatory properties that support overall cellular health.
The world has been spiked, and we are living through one of the most extraordinary chapters in medical history. My team and I are committed to advancing research and delivering real, evidence-informed solutions to help people heal and rebuild their health. With the same conviction that drove me to speak out before this crisis began, I continue to channel that energy towards the recovery and restoration of health and the rule of law.
We operate on a lean budget and depend on your generosity to continue this essential mission. Every contribution fuels our work—developing science-based detoxification formulas, sharing life-saving knowledge, and maintaining one of the most active public health education platforms online, with over 1,200 posts published in just four years. To contribute, consider a paid Substack subscription or making a one time donation today.
Together, we can bring truth, healing, and accountability back to medicine.